Denis Chem Vision :
To be a Leading Pharmaceutical company recognized by the society and its stake holders for its Best business practices and its contribution in saving lives.
Denis Chem Mission :
To deliver High quality Medical products in multiple therapy segments by ensuring quality in every drop. To ensure shareholder returns by constantly Innovating ,Transforming and Delivering. To Make some one else’s life better.
Major Corporate Events
| Year | Important Events |
| 1980 | Incorporated as a Private Limited Company on July 15, 1980 |
| 1982 | Conversion into Public Limited Company |
| 1982 | Initial Public Offer of our Company |
| 1984 | Rights Issue of 70,000 equity shares at a issue price of ` 10/- per share |
| 1989 | Rights Issue of 1,17,728 equity shares at a issue price of ` 10/- per share |
| 1991 | Production capacity increased to 8 million glass bottles per annum |
| 1994 | Production capacity again increased to 20 million glass bottles for I.V. Fluids |
| 1995 | Further Public Offer of 1,80,750 equity shares |
| 2006 | Company forayed into manufacturing of IV fluids in PP bottles using BFS technology |
| 2013 | Issue of Bonus Shares in the ratio of 1:1 |
| 2014 | Listing of Equity Shares of our Company on BSE Limited under the Direct Listing Norms |
| 2014 | Sucessfuly Completed The Rights Issue for the project of Aqua Pulse |
| 2014 | Sucessfuly Launched Aqua Pulse Pan India |
We are constantly innovating, transforming and delivering company with an ambition of setting bench marks in various aspects of business and having a focus on our contribution of saving lives and making someone else’s life better.
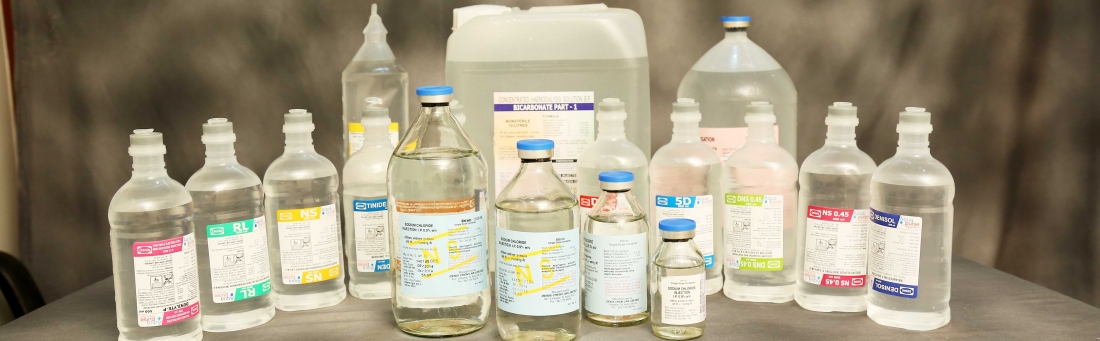
We are a Public Limited Pharmaceutical Company incorporated in 1982. We started commercial production for sterile Intravenous injectibles in 1984. Since more than 30 years we have been actively delivering high quality sterile Intravenous injectibles and contributing in saving lives. We Manufacture and market sterile Intravenous injectibles in Both Glass and Plastic Bottles with capacity of 810 million bottles per Annum.
Company’s manufacturing facilities are located in a pollution free environment near Ahmedabad, Gujarat. The Manufacturing facilities are located on a land area of about 14000 sq. metres, the built up area for the manufacturing operations is about 7000 sq.m.
Our manufacturing facilities have been approved by the Foods and Drugs Control Administration Authority (FDCA), Gujarat & WHO GMP. We have also been approved by international bodies, includes FDCA Ivory Cost, Ministry of Health-Vietnam, Ministry of Health-Combodia.
Apart from these certifications our manufacturing facilities are ISO 9001-2008 certified.
We offer wide range of sterile intravenous injectables for human and veterinary consumption. Our Company’s product offerings comprise of more than seventy (70) products sold across Seventeen (17) states in India.
We also export sterile intravenous medicines to countries like Sri Lanka, Nepal, Combodia, Vietnam, Somalia, Ivory Cost etc.
In addition to manufacturing our own products which we sell under our own brands, we do manufacture products on contract basis for third parties including certain multinational pharmaceutical companies who market such products under their own brands.
Our manufacturing facility has an installed capacity to manufacture 240 Million plastic bottles 258 million glass bottles and 312 million of Aqua Pulse bottles per Annum.
The company has a fully equipped in-House Quality Control Laboratory having different inter-departments for Chemical Testing, Physico-Chemical Testing, Microbiological Testing, Pyrogens Testing, Sterility Testing and Toxicity Testing. All raw materials, packaging materials, in process testing and finished stocks are tested in-house.